What is a Device Master Record?
Table of contents
The Device Master Record (DMR) is a regulatory requirement for Medical Device product development. In short, a DMR contains all the information needed to manufacture your product. This includes, but is not limited to:
- The approved, released specifications for the finished device and details of all components, software, any applicable formulation, and composition.
- Production process specifications, including production methods, process details, measurement and test equipment, operating and test procedures, and environmental specifications.
- Quality Assurance test, measurement and operating procedures, quality assurance and quality control acceptance criteria, details on all quality-related testing and measurement equipment.
- Device packaging specifications and operating procedures.
- Labelling specifications, procedures, and examples.
- Operating procedures and methods applicable to product installation, maintenance, and service.
Design History File (DHF) – The DHF includes a full history of the device design, from the establishment of user needs to the reports of your design reviews and the device specifications created in the design transfer process.
Device History Record (DHR) – This record contains information about specific batches, lots, or units of product that you produce. It is meant to facilitate traceability of your products throughout their life cycle and to help with root cause analysis by connecting customer feedback and audit results to specific batches of product.
What is Device Master Record Management?
The DMR lets companies quickly assess the overall impact of any change to the manufacturing process. Device Master Record Management enables Design Engineers and Manufacturing Engineers to manage the engineering design process for Life Science apps. It helps with audit trails and authentication controls for every step in the design and release process.
Device Master Record Application Functions
Your teams can use the DMR View to see all product parts, the Bill of Material (BOM) structure and its connected specifications, and process documents associated with the engineering design. The list shows relationships between items, enabling you to add, remove, and reorganize items as needed.
This view ensures a consistent manufacturing process by associating product documents, and reference lists within the DMR/BOM. The DMR View lets you specify which plants use a part, document, or reference list. There are few major areas where DMRs provides users some great help:
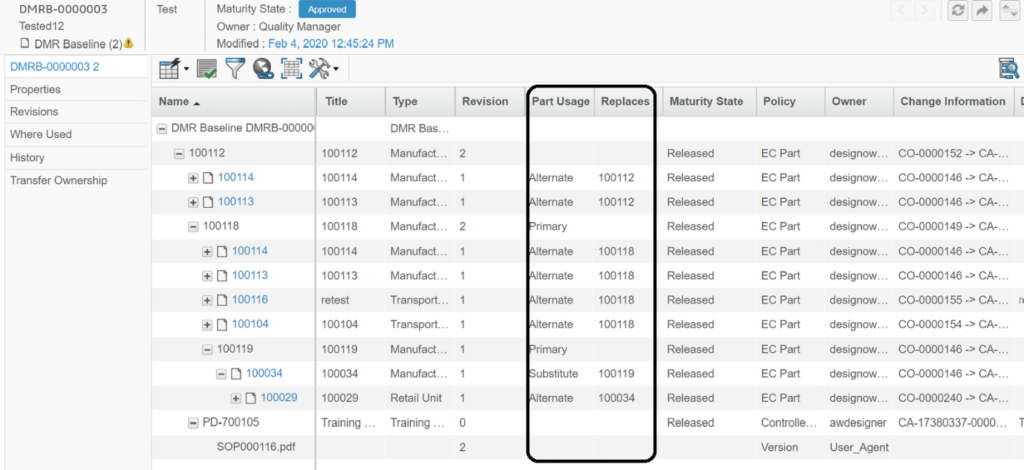
DMR Baselines – Baselines are used to manage the approval of Device Master Records. Quality Managers are responsible for making these decisions. Creating DMR Baselines is basically a process of recording current DMR Structures. Baselines approval process also provides the provision to add Tasks and assign them to respective stakeholders for having a better control on decision making process.
DMR Snapshots – DMR snapshot is recording DMR Baselines at a specific point in time. They cannot be refreshed or revised. However, any user can create DMR Snapshots. Snapshots are majorly used for comparisons to check how things have changed from different times.
DMR Packages – Life Sciences customers request their suppliers or third-party vendors to handle design or manufacturing activities. To help enable this request, they share with their suppliers or vendors a package that contains all the necessary designs, BOMs, and other documents.
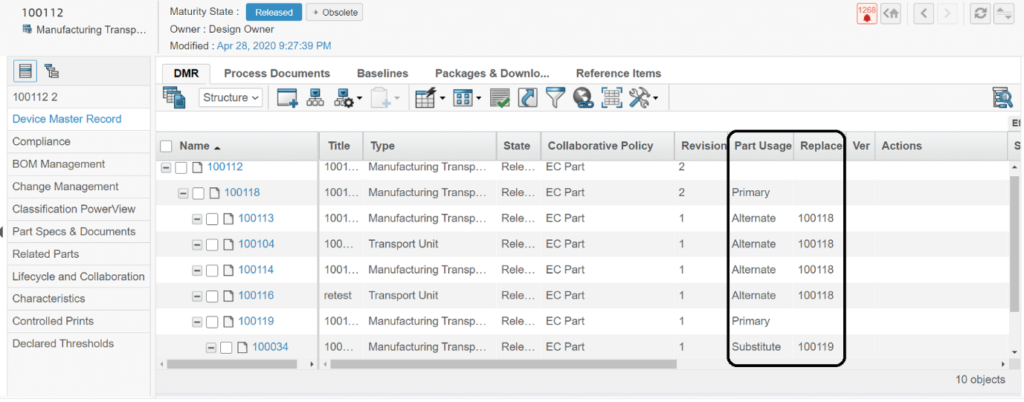
Alternates and Substitutes for a Part
An Alternate Part is a component that is a suitable replacement for another part in every assembly in which the original part occurs. Companies use alternate parts when multiple vendors can supply parts that serve the identical function and fit.
A Substitute Part is a component that is a suitable replacement for another part in only one assembly in which the original part occurs.
Medical Devices Manufacturer Challenges
Life Sciences industries are often required to send out Baseline Structures to their suppliers. Filtering a number of different parts and connected specifications in a BOM but also, the connected Alternates and Substitutes connected to those parts of the BOM. Currently, users are not supported with a unified view of all Alternates and Substitutes to a part. So, users must navigate down to individual parts to locate their corresponding Alternates and Substitutes. And DMR Baselines for different Plants are only supported through MBOM and Manufacturing Parts.
TECHNIA’s Solution
Many manufacturers want to see a unified view for alternates and substitutes of parts associated to a structure. They also use this view to coordinate with various suppliers. The Baseline structures can then be used by suppliers to see different versions of the same BOM depending upon the chosen components.
So, we’ve customized the BOM View to include Alternates and Substitutes during the design phase of BOM.
Similarly, we’ve customised the DMR, DMR Baseline Creation, Snapshot Creation and DMR Package Creation views to include all relevant information in one place.
This provides users the option of applying an Engineering Bill of Material (EBOM) or simulating an MBOM during the design phase.
New Part structures that have an abstract Manufacturing Part can hold some Design Parts and Manufacturing Part Material together, along with their Alternates and Substitutes.
Plant-specific parts and BOM structure are shown within multivalued attribute plants. So, now the user can create an MBOM for multiple plants, and Quality Managers can easily decide which parts from a specific plant can be sent for approval using Baselines.
Types Structure
TECHNIA’s Life Sciences customers use DMR Baselines containing all Alternates and Substitutes for their entire BOM structure and associated Part Specifications. This enables their Quality Managers to see this view and make decisions on Approving and Rejecting them accordingly.
Out of the Box (OOTB) Baselines support different types of configuration for DMR Baselines, namely: MBOM, EBOM and Both.
Since our solution does not use MBOM, we have limited Baseline configuration to “both”, “only” and “any change” so that approved Baselines can be accommodated while revising the “Approved Baseline”. And changes to “In-Work Baselines” can be accommodated by refreshing Baselines. As with DMR Baselines, DMR Snapshots are also customized to show Alternates and Substitutes for the BOM Structure.
Device Master Record Management also enables Quality Managers to choose the content of the package they wish to send to their suppliers. And our solution provides the choice between Alternates and Substitutes for respective BOM structures. Please note that DMR Packages are always created from a DMR Baseline or a Snapshot.
There are comparisons available in the raw application which allow users to compare DMR Structures of different parts and to compare various snapshots or baselines. And this solution also supports the comparison of Alternates and Substitutes.
BOM View
DMR View
DMR Baseline View
DMR Compare View
Device Master Record Management Benefits
Device Master Record Management helps users to maintain different packages to be send to different suppliers and maintain discretion among them. It reduces the scope of error due to redundant information coming from various system and makes job easier for Life Sciences industry. Our solution enhances the capabilities of the Device Master Record and provides the Life Sciences Industries to investigate the DMR Structures in new light without having to depend on MBOM Module.
It enables the users to view Alternates and Substitutes attached to different parts in all the DMR Views.
It also provides the provision to add the DMR Baselines as Deliverable to a DHF project to make it visible to the relevant Project/Task owner and get an overall view of the product and its variants.
Browse webinars on demand from the Life Sciences PLM Innovation Forum to learn more about digitalization in the Life Sciences industries!